Equine (Horse) Vet Medicines
Equine (Horse) Injectable Veterinary Products
ABXOL - Ambroxol Hydrochloride Injection
Ambroxol Hydrochloride B.P 6 mg
Water For Injection B.P q.s.
specific indications include :
Chest Infections : acute and chronic, bronchopneumonia, catarrhal rhinitis, strangles, post-viral cough.
Uterine infections : pyometron,mucometron.
Dogs : 0.6 mg/kg (1 ml/kg) bodyweight twice daily.
Administer ABXOL by Intravenous Injection.

B-COX - Vitamin B Complex Injection
Thiamine Hydrochloride (Vitamin B1) BP 10 mg
Riboflavin Sodium Phosphate(Vitamin B2) BP 0.1 mg
Pyridoxine Hydrochloride (Vitamin B6) BP 1 mg
Nicotinamide BP 10 mg
D-Panthenol USP 0.5 mg
Benzyl Alcohol BP 2% v/v
(as preservative)
Water for Injection BP q.s.
B COMPLEX VITAMINS:
Maintains nerve functions, skin, hair and muscle tone
Are vital to all energy supply system.
Are essential to red blood cell formation.
Help Reduce the effects of stress.
It is used for supportive purposes besides principal treatment of absorption disorders, inflammatory diseases, acute an chronic infections and related recovery period during oral antibiotic and sulfonamides administration.
SPECIES THERAPEUTIC DOSE
Cattle, Horses 15-30 ml
Calves, Heifer 10-15 ml
Calves, Foals 5-10 ml
Sheep, Goats 5-10 ml
Swine 2-5 ml
Dogs 1-5 ml
Cats 1 ml

BUTACIN - Butaphosphan and Cyanocobalamin Injection
Butaphosphan 100 mg
Cyanocobalamin BP 50 mcg
Methyl Hydroxy Benzoate BP 0.1% w/v
Water for Injection BP q.s.
BUTACIN additionally supports muscular physiology, the treatment of infertility, and tetany and paresis as an adjunct to calcium and magnesium therapy.
Horses and cattle : 10.0 - 25.0 ml.
Foals and calves : 5.0 - 12.0 ml.
Sheep and goats : 2.5 - 8.0 ml.
Lambs : 1.5 - 2.5 ml.
Pigs : 2.5 - 10.0 ml.
Gilts : 1.0 - 2.5 ml.
Dogs : 0.5 - 5.0 ml.
Cats, fur-bearing animals : 0.5 - 2.5 ml
If necessary, repeat the treatment next day.
For birds Butacin added to the drinking water at the rate of:
Chicks and young 1.0 - 1.5 ml to 1 liter
Broilers and laying hens 2.0 - 3.0 ml to 1 liter and fed within 4 - 5 days.
Milk : 0 days
Egg : 0 days

CIT-C - Ascorbic Acid Injection
Ascorbic Acid (Vitamin C) B.P. 500.0 mg
Methyl Hydroxybenzoate B.P. 0.8 mg
(As Preservatives)
Propyl Hydroxybenzoate B.P. 0.2 mg
(As Preservatives)
Water for Injection B.P. q.s.
Vitamin C supplement for horses and dogs.
- Treatment of vitamin C deficiency.
- Adjuvant therapy for asthenia, notably in cases of infection or stress.
Horses : 5-10 mlDogs : 1-2 ml
Administer twice weekly or as directed by a veterinary surgeon.

CLOSIN 5% - Closantel Injection 5% w/v
Closantel 50 mg
Benzyl alcohol B.P 2% v/v
Propylene glycol B.P q.s.
CLOSIN is indicated for the effective treatment and control of the following parasites Gastrointestinal roundworms (adult and fourth-stage larvae), Ostertagia ostertagi, O. lyrata, Haemonchus placei, Trichostongylus axei, T colubriformis, Cooperia oncophora C punctata, C pectinata, Bunostomum phlebotomum, Nematodirus helvetianus (adult only), Napathiger (adult only), Oesophagostomum radiatum.
Lungworms (adult and fourth-stage larvae) : Dictyocaulus viviparous.
Liver flukes (adult and juvenile) (6-8 weeks).
Cattle grub (parasitic stages) : Hypoderma bovis, H lineatum.
Sucking Lice : Linognathus vituli, Haematopinus eurysternus, Solenopotes capillatus.
Mange Mites (Cattle scab) : Psoroptes ovis, Sarcoptes scabiei var. bovis.

CLOSIN 10% - Closantel Injection 10% w/v
Closantel 100 mg
Benzyl alcohol B.P 4 % v/v
Propylene glycol B.P q.s.
CLOSIN 10 % is indicated for the effective treatment and control of the following parasites - Gastrointestinal roundworms (adult and fourth-stage larvae), Ostertagia ostertagi, O. lyrata, Haemonchus placei, Trichostongylus axei, T colubriformis, Cooperia oncophora C punctata, C pectinata, Bunostomum phlebotomum, Nematodirus helvetianus (adult only), Napathiger (adult only), Oesophagostomum radiatum.
Lungworms (adult and fourth-stage larvae): Dictyocaulus viviparous.
Liver flukes (adult and juvenile) (6-8 weeks).
Cattle grub (parasitic stages): Hypoderma bovis, H lineatum.
Sucking Lice: Linognathus vituli, Haematopinus eurysternus, Solenopotes capillatus.
Mange Mites (Cattle scab): Psoroptes ovis, Sarcoptes scabiei var. bovis.
1ml / 50kg of bodyweight by Subcutaneous Injection only


CLOX-M 3 g. - Amoxicillin & Cloxacillin Powder For Injection 3 g
1. Amoxicillin Sodium B.P equi. to
Anhydrous amoxicillin 1.5 g
Cloxacillin Sodium B.P equi. to
Cloxacillin 1.5 g
2. Sterilised Water for Injection I.P 10 ml 1- Ampoule
Large animals : 7 mg/kg body weight or 1 ml of for 40-45 kg body weight (Practical Dosage) once daily for up to five days.
Administration : Intramuscular
Small animals : 15-25 mg/kg body weight once daily for up to five days.
Administration : Intramuscular
The reconstituted solution should be used immediately after preparation.
Do not allow to freeze.
Milk : 48 hours
Sheep & Goats : Meat : 35 days
Pig : Meat : 14 days

CLOX-M 4 g. - Amoxicillin & Cloxacillin Powder For Injection 4 g
1. Amoxicillin Sodium B.P. equi. to
Anhydrous amoxicillin 2 g
Cloxacillin Sodium B.P. equi. to
Cloxacillin 2 g
2. Sterilised Water for Injection I.P. 20 ml 1- Ampoule
Large animals : 7 mg/kg body weight or 1 ml of 40-45 kg body weight (Practical Dosage) once daily for up to five days.
Administration : Intramuscular
Small animals : 15-25 mg/kg body weight once daily for up to five days.
Administration : Intramuscular
The reconstituted solution should be used immediately after preparation.
Do not allow to freeze.
The withdrawal periods after the last treatment are as follows :
Cattle : Meat : 21 days
Milk : 48 hours
Sheep & Goat : Meat : 35 days
Pig : Meat : 14 days

COMIN - Cyanocobalamin Injection
Cyanocobalamin B.P 500 mcg
Benzyl alcohol B.P 1.5% v/v
Water For Injection B.P q.s.
Dogs : 1-2 ml
Administer by Intramuscular Injection regularly twice weekly advised by a Veterinary surgeon.

CRAMINE - Chlorphenamine Injection B.P. 10 mg/ml
Chlorphenamine Maleate B.P. 10 mg.
Phenylmercuric Nitrate B.P. 0.002% w/v.
(As Preservative)
Water for Injection B.P. q.s.
2. Rhinitis, dyspnoea, pulmonary emphysema, pulmonary oedema, asthama and hay fever.
3. Eczema, dermatitis, urticaria, insect bite, tail eczema in Horses, toxic hoof corns.
4. Ruminal atony, tympany and bloat in ruminants.
5. Puerperal toxaemia and secondary placental retention, acute septic metritis and gangrenous mastitis.
6. Reactions and other drug allergies and anaphylactic shock.
Do not use in rental and hepatic insufficiency.
Sheep & Goat : 0.5 - 2 ml. By Intramuscular route.
Canine : 0.5 - 2 ml
Poultry : 0.5 - 1 ml. By Intramuscular route.
Or as directed by Physician.

CULANATE - Calcium Levulinate, Vitamin D3 & Vitamin B12 Injection
Meat : Nil


DEXACIN VET - Dexamethasone Sodium Phosphate Injection B.P. 4 mg/ml
Dexamethasone Sodium Phosphate B.P.
eq. to Dexamethasone Phosphate 4 mg.
Methyl Hydroxybenzoate B.P. 0.15% w/v
(As Preservative)
Propyl Hydroxybenzoate B.P. 0.02% w/v
(As Preservative)
Water for Injection B.P. q.s.
Treatment of anti-inflammatory or allergic conditions.
Cattle : Induction of parturition. Treatment of primary ketosis (acetonaemia).
Horses : Treatment of arthritis, bursitis or tenosynovitis.
Do not use in animals suffering from gastrointestinal or comeal ulcers or demodicosis. Do not administer Intra-articularly where there is evidence of fractures, bacterial joint infections and aseptic bone necrosis. Do not use in known cases of hypersensitivity to the active substance, to corticosteroids and to any other ingredient of the product.
SPECIES DOSAGE
Horse, Cattle, Pigs 0.06 mg/kg Body Weight
Dogs, Cats 0.1 mg/kg Body Weight
DEXACIN can be administered by Intravenous or Intramuscular injection in Horses and by Intramuscular Injection in Cattle, Pigs, Dogs and Cats.
Meat : 8 days

DEXACIN-F - Dexamethasone with Furosemide Injection
Furosemide B.P 20 mg
Dexamethasone Sodium Phosphate B.P 0.63 mg
Phenol B.P 0.5% v/v
Water for Injection B.P q.s.
Duration of treatment may vary according to each clinical case; usually 2 doses with a 24 hr interval are sufficient.
Milk : 2 days

DEXAZIDE - Dexamethasone with Hydrochlorothiazide Injection
Dexamethasone sodium phosphate B.P 0.50 mg
Hydrochlorothiazide B.P 50.00 mg
Propylene glycol B.P 0.15 ml
Benzyl alcohol B.P 0.01 ml
Water for Injection B.P q.s.
- Congestion and oedema of the udder.
- Early persistant lactation edema.
- Pulmonary congestion and edema.
- Edematous infiltration of surgical wounds.
- Edema in allergic conditions.
HORSES :
- Generalised congestion and oedema
- Oedema of sheath Anasarca
- Oedema in allergic conditions
Cattle and ault Horses :
Preventative treatment : 10 ml daily for 3 days.
Curative treatment :
Congestion and mild oedema : 10 ml daily for 2 or 3 days
Congestion and severe oedema : 20 ml daily for 2 days and 10 ml the third day.
Foals - Calves : 2 ml per 40 - 50 kg bodyweight daily for 3 days
Milk : 7 days
Treated Horses may never be slaughtered for human consumption.


DIMINACIN R.T.U. - Diminazene Aceturate 7% Injection
Diminazene Aceturate 70 mg
Phenazone B.P (antipyrine) 375 mg
Water for Injection B.P q.s.
Administration to animals with an impaired renal and/or hepatic function.
Do not exceed a total dose of 4 g of active ingredient in any animal (or 56 ml of DIMINACIN).
Milk : 3 days


DRAMINE - Diphenhydramine (Antihistamine) - Vitamin B & C Injection
Diphenhydramine hydrochloride 6.25 mg
Thaimine HCL (vit. B1) 20 mg
Ascorbic acid (vit C) 112.4 mg
Glucose 100 mg
Water for Injection B.P q.s.
Pigs : Especially oedema disease, gastric ulcer, Pruritus and all disturbances of metabolism.
Dogs & Cats : Allergies, diseases of the skin, especially in conjunction with itch.
Dogs & Cats : 1 to 5 ml
Sheep & Goats : 5 to 10 ml
Calf & Pigs : 5 to 10 ml
Cattle & Horses : 20 to 50 ml


FLUXANE - Flunixin Meglumine Injection 5% w/v
Flunixin meglumine USP
equi. to flunixin 50 mg
Phenol B.P
0.5% w/v
(As Preservative)
Water for Injections B.P q.s.
Horses : For the alleviation of inflammation and pain associated with musculoskeletal disorders. It is recommended for the alleviation of visceral pain associated with colic.
The cause of the acute inflammatory condition should be determined and appropriate concomitant therapy initiated.
Horses : The recommended dose for musculoskeletal disorders in Horses is 1.1 mg per kg of bodyweight once daily. Treatment may be given by Intravenous or Intramuscular Injection and repeated for up to five days. Studies show that onset of activity is within two hours. Peak response occurs between 12 and 16 hours and duration of activity is 24-36 hours. The recommended dose for the alleviation of pain associated with equine colic is 1.1 mg per kg of bodyweight.
Intravenous administration is recommended for prompt relief. Clinical studies show pain is alleviated in less than 15 minutes in many cases. Treatment may be repeated when signs of colic recur. During clinical studies approximately 10% of the Horses required one or two additional treatments. The cause of colic should be determined and treated with concomitant therapy.
Milk : 36 days
Horses : Meat : 7 days
All target species : 22 days

FUTIC - Furosemide Injection BP 5% w/v
Furosemide Sodium B.P
equi.to Furosemide 50 mg
Benzyl Alcohol B.P 1% v/v
(as preservative)
Water for Injection B.P q.s.
Do not use concurrently with aminoglycoside antibiotic treatment.
Cattle : 0.5 - 1 mg/kg bodyweight.
Horses : 0.5 - 1 mg/kg bodyweight once or twice daily at 6 to 8 hour intervals.
Administration: Intramuscular or Intravenous route
Cattle : Meat & Milk : 48 hours.


I-CARE - Levocarnitine Injection
Levocarnitine B.P 200 mg
Water For Injection B.P q.s.
- Muscle levels of Levocarnitine determine the exercise capacity of muscles.
- Levocarnitine forms the essential transport system for use of fats as an energy source.
- Levocarnitine may help delay muscle fatigue and improve endurance.
- Levocarnitine is essential for normal heart function.
- Performance Horses recover more effeciently when supplemented with Levocarnitine.
Levocarnitine is an amino acid which helps transport fats into muscle cells. Levocarnitine is essential in the transport of fats into muscle cells for production. The muscle levels of Levocarnitine determine the exercise capacity of muscles. By using fats as energy for muscle contractions, the body is glycogen and delaying the accumulation of lactic acid. Levocarnitine delays muscle fatigue by reducing lactic acid formation and improves performance and endurance. Levocarnitine forms an essential part of the transport system which moves fatty acids into the mitochondria (cell furnaces) for energy production. It thus acts as a buffer by inhibiting lactic acid buildup in muscles, helping to delay fatigue and prevent Tying Up. Demand for Levocarnitine in heavily exercising Horses is often not met from the diet, as large amounts are consumed during exercise. Supplementing with Levocarnitine results improved energy supply, increased use of fatty acids as an energy source, decreased lactate buildup and a significant increase in maximum work output.
Dogs.1 ml/10 kg bodyweight.
Administer 2-3 time weekly or as directed by veterinary surgeon


I-CLOR - Dichloro Acetic Acid Injection
Dichloro-acetic acid 120 mcg
Sodium gluconate USP 250 mg
Water For Injection BP q.s.
Supplementation with dichloroacetic acid (I-CLOR) results in activation of the enzyme pyruvate dehydrogenase, leading to a reduction in the rate of lactic levels results in a reduction in pH which contributes to muscle fatigue and decreased muscle performance. Supplementation with I-CLOR has been shown to reduce lactic acid accumulation during exercise, and produce a significant delay in muscle fatigue. The pathogenesis of Exertional Rhabdomyolysis(”Tying Up”) in Horses is related to lactic acidosis during exercise, and associated low muscle pH. Sodium bicarbonate and dimethylglycine (DMG), which may help to reduce lactic acid accumulation during exercise, have been used to assist in the prevention of Tying Up in horses. Similarly, I-CLOR results in a reduction in the rate of lactate accumulation, and a delay in the onset of muscle fatigue.

I-LIC - Folic Acid Injection
Folic acid B.P 15 mg
Water For Injections B.P q.s.
Dogs : 1 - 2 ml
Administer by Intramuscular Injection regularly twice weekly advised by a Veterinary surgeon.

IMIX - Imidocarb Dipropionate Aqueous Injection Solution
Imidocarb Dipropionate 120 mg
Benzyl Alcohol B.P 2.0% w/v
Water For Injections B.P q.s.
Cattle : 1 ml per 100 kg bodyweight. In treating mixed infections due to Anaplasma and Babesia, administer 2.5 ml per 100 kg body weight.
Sheep : 0.5 ml per 50 kg bodyweight.
Horses : 2 ml per 100 kg bodyweight. (Intramuscular injection is preferred). In most cases, a single dose will effect a complete cure, but treatment of B. equi infections in Horses may require 2 doses at an interval of 24 hours.
Dogs : 0.25 ml per 10 kg body weight. In treating mixed infection due to Ehrlichina and Babesia, administer 0.5 ml per 10 kg bodyweight, 2 doses at an interval of 14 days.
It is advised to screen the blood after one treatment for presence of the parasite. Repeat treatment for presence of the parasite. Repeat treatment if the test is positive.
Milk : 7 days.


IMOLY 5% - Oxytetracycline Hydrochloride Injection 5%
Oxytetracycline Hydrochloride B.P 50 mg
Propylene Glycol B.P q.s.
A transient swelling may be observed following Intramuscular administration in Horses and Subcutaneous administration in Dogs.
The use of tetracyclines during the period of tooth and bone development, including late pregnancy, may lead to discolouration.
Not to be used in Sheep producing milk for human consumption.
Not to be used in Horses intended for human consumption.
Small Animal : 5-10 mg Oxyteracycline per Kg Body Weight for 3 to 5 days by IM or IV Injection
The withdrawal periods after the last treatment for Cattle, Sheep are :
Cattle : Milk : 6 days Meat : 35 days
Sheep : Meat : 14 days

IMOLY 10% - Oxytetracycline Injection 10%
Oxytetracycline Dihydrate B.P eq. to
Anhydrous Oxytetracycline 100 mg
Propylene Glyco B.P q.s.
A transient swelling may be observed following Intramuscular administration in Horses and Subcutaneous administration in Dogs. The use of tetracyclines during the period of tooth and bone development, including late pregnancy, may lead to discolouration. Photodermatitis may occur after treatment if exposure to intense sunlight.
Not for Intravenous administration in Dogs or Cats. It is not recommended to administer bacteriostatic and bactericidal antimicrobials concurrently.
Full-grown animals : 1 ml per 10 - 20 kg body weight for 3 - 5 days.
Young animals : 2 ml per 10 - 20 kg body weight for 3 - 5 days.
Do not administer more than 20 ml in Cattle, more than 10 ml in Swine and more than 5 ml in Calves, Goats and Sheep per Injection site.
The withdrawal periods after the last treatment for Cattle, Sheep, Camel, Goat are :
Cattle & Camel : Milk : 6 days Meat : 35 days
Sheep & Goat : Meat : 14 days

IMOLY 20% LA - Oxytetracycline Injection Long Acting 20%
Oxytetracycline Dihydrate B.P
eq. to Anhydrous Oxytetracycline 200 mg
in 2-Pyrrolidone Vehicle system q.s.
For the treatment and control of pasteurellosis and pneumonia caused by oxytetracycline sensitive organisms and as an aid in the treatment of infectious bovine keratoconjunctivitis (New Forest Disease) due to oxytetracycline sensitive strains of Moraxeila bovis. This product may also be of value for foul-in-the-foot.
The use of this product during the period of tooth development including late pregnancy may lead to tooth discoloration.
General : 1 ml per 10 kg body weight.
This dosage can be repeated after 48 hours when necessary.
Do not administer more than 20 ml in Cattle, more than 10 ml in Swine and more than 5 ml in Calves, Goats and Sheep per Injection site.
Species Meat withdrawal periods
Cattle & Sheep 28 days
Pigs 35 days


IMOLY 30% LA - Oxytetracycline Injection Long Acting 30%
Oxytetracycline Dihydrate B.P
eq. to Anhydrous Oxytetracycline 300 mg
in 2-Pyrrolidone Vehicle system q.s.
Cattle : For the treatment and control of pasteurellosis and pneumonia caused by Oxytetracycline sensitive organisms and as an aid in the treatment of infectious bovine keratoconjunctivitis (New Forest Disease) due to Oxytetracycline sensitive strains of Moraxeila bovis. This product may also be of value for foul-in-the-foot.
Pigs : For the treatment of pneumonia caused by Pasteurella.
Sheep : For the control of enzootic abortion and pneumonia caused by Oxytetracyclinesensitive organisms. This product may be used as an aid in the treatment of foot rot, acute, severe mastitis and infectious ovine keratoconjuctivitis (pink-eye).
The use of this product during the period of tooth development including late pregnancy may lead to tooth discoloration.
Deep intramuscular use only in large & small animals and subcutaneous in poultry.
In large & small animal & poultry.
1ml / 10kg body wt.
0.25ml / 1kg body wt.
Cattle and sheep : 28 days.
Swine : 14 days.
For meat (high dose):
Cattle : 35 days.
Sheep and swine : 28 days.
For milk :
Cattle : 10 days.
Sheep : 8 days.


INCEFT - Ceftiofur Sodium Powder For Injection
Ceftiofur Sodium eq. to Ceftiofur 1 g
For Intramuscular Injection in Cattle, Buffaloes, Sheep & Goat. It can be used in lactating dairy animals.
- Genital infections of bovine (acute metritis, cervicitis, prolapsed related to retention of placenta cases) associated with Arcanobacterium pyogenes, Fusobacterium necrophorum and bacteroids spp.
- Respiratory diseases of Cattle, Buffalo, Sheep and Goat (Shipping fever, pneumonia) associated with Pasteurella haemolytica, Pasteurella multocida & Haemophilussomnus.
- Acute interdigital necrobacillosis (foot rot, pododermatitis) caused by Fusobacterium and Bacteroides.
Buffalo : 2 to 2.4 mg/kg b.wt. by IM use only, once daily for 3-5 days.
Dog : 2.2 mg/kg b.wt. by SC use only, once daily for 5 to 14 days.
Camel : 2.2 mg/kg b.wt. by IM once daily for 3-5 days.
Horse : 2.2 to 4.4 mg/kg b.wt. or 1 to 2 ml (after reconstitution) /22.72 kg b.wt. by IM use only. once daily for 3 to 5 days.
Swine : 3 to 5 mg/kg b.wt. or 1 to 1.7 ml (after reconstitution) / 16.66 kg b.wt. by IM use only, once daily for 3 to 5 days.
Chicken (day-old chick) : 0.08 to 0.2 mg/Chick, SC use only in the neck region (1 g vial after reconstitution with 20 ml water for injection will treat approximately 5,000 to 12,500 day-old chicks)
Milk : Nil | Meat : 4 days
For Sheep & Goat :
Milk : Nil | Meat: Nil

IROFLOX SA - Enrofloxacin Injection
Enrofloxacin USP 100 mg
Benzyl Alcohol B.P 1.5% v/v
[as preservative]
Water for Injection B.P q.s.
Mastitis caused by susceptible microorganisms and renal infection.
IROFLOX SA is effective against the following microorganism : Mycoplasma spp., E.coli, Salmonella spp., Haemophilus spp., Pasteurella spp., Klebsiella spp., Proteus spp., Vibrio parahaemolyticus and Staphylococcus aureus.
As directed by the veterinarian. OR 1 ml per 20-40 kg body weight depending upon the severity of injection. (2.5-5 mg of Enrofloxacin 1kg body weight) the second administration should be administered after 72 hours, if required.
ROUTE :
For I.M./SC/Slow I.V. Use.
The withdrawal periods after the last traetment are:
Cattle:
For Intravenous route : Meat : 4 days
Milk : 72 hours
For Subcutaneous route : Meat : 14 days
Milk : 84 hours

K-GEN 10% - Gentamicin Injection BP
Gentamicin Sulphate B.P
eq. to Gentamicin Base 100 mg
Methyl Hydroxybenzoate B.P 0.18% w/v
(As Preservative)
Propyl Hydroxybenzoate B.P 0.02% w/v
(As Preservative)
Water for Injection B.P q.s.
General: Twice daily 1 ml per 20 - 40 kg body weight for 3 days.
- For meat : 7 days.
- For milk : 3 days.


MELOXIN-I - Meloxicam Injection 5 mg/ml
Meloxicam B.P 5 mg
Benzyl alcohol (as preservative) B.P 2% v/v
Water for Injection B.P q.s.
- Pneumonia
- Broncho-Pneumonia
- Pleuritis
- Arthritis
- Synovitis
- Myositis
- Tendonitis
- Laminitis
- Bursitis
- Metritis
- Prolapse of uterus
- Mastitis
- Otitis
- Pharyngitis
- Postoperative Pain
- Pyrexia of Unknown Origin
- Pain and fever associated with inflammation
1ml / 10kg (Respiratory Inflammation & Prolapse)
1ml / 25kg (Other Indications)
Dog : 1ml / 10 kg
Meat and offal : 15 days
Milk : 5 days
Horses & Swine :
Meat and offal : 5 days


MELOXIN-20 - Meloxicam Injection 20 mg/ml
Meloxicam B.P 20 mg
Benzyl alcohol B.P 2% v/v
Water for Injection B.P q.s.
Do not use in case of hypersensitivity to the active substance or to any of the excipients. For the treatment of diarrhoea in Cattle, do not use in animals of less than one week of age.
Meat : 15 days
Milk : 5 days

MICIX - Amikacin Injection 250 mg/ml
Amikacin Sulphate B.P
Equivalent to Amikacin base 250 mg
Methyl hydroxy benzoate B.P 0.18% w/v
Propyl Hydroxy benzoate B.P 0.025% w/v
Water for Injection B.P q.s.
7 mg/kg IV, IM q8h
22 mg/kg IV, IM q24h [once daily dosing]
Horses:
22 - 25 mg/kg IV q24h for neonates
5 mg/kg IV q24h for older foals and adults
Adults : 6.6 - 10 mg/kg IM, IV q24h [once daily dosing]
Mastitis / Endometritis : 8 ml of MICIX Inj mixed with 200 mL 0.9%
Sodium Chloride Injection, daily for 3 - 5 consecutive days
[intrauterine/intramammary use]
POULTRY :
10 mg/kg b.wt. S/C or I/M injection
Meat : 28 days


NEROCIN - Injection of Methylcobalamin, Vitamin B6 & Nicotinamide
Methylcobalamin 500 mcg
Pyridoxine Hydrochloride B.P 50 mg
Nicotinamide B.P 50 mg
Benzyl Alcohol B.P 2% w/v
(as preservative)
Water for Injection B.P q.s.
Overanges of vitamins are added to compensate loss on storage.
Debility and exhaustion.
Anorexia, Fatigue.
To prevent anemia and normal RBC production.
As a supportive therapy with antimicrobials and antiparasitic treatment.
Dog : 2 to 3 ml IM or IV daily
For normal Condition : 3 days
For Acute Condition : 7 days
Meat : Nil

NOLON - Triamcinolone Injection BP 6 MG/ML
Triamcinolone Acetonide BP 6 mg
Benzyl Alcohol BP 0.9% w/v
Water for injection BP Q.S.
- Bovine Ketosis
- Arthritis
- Tenosynovitis and related disorders
- Dermatological Disorders including pruritus
- Allergic reactions and dermatoses
- Haematoma
- Inflammatory conditions
- Pregnancy toxaemia in ewes/sheep
2.5 mg to 10 mg given in a single intramuscular injection.
| Animal | Intra-Articular/Intrasynovial | Intramuscular/Sub-cutaneously in Arthritic or Allergic conditions |
|---|---|---|
| Horses/Cattle/Camel | 6 to 18 mg | 12 to 20 mg |
| Dogs & Cats | 1 to 3 mg | 0.1 to 0.2 mg per kg |
Meat : Nil


PAXON - Parvaquone injection 150 mg/ml
Parvaquone 150 mg
Sorbitan Mono Oleate B.P 100 mg
(as preservative)
Oil base q.s.
Calves : 1 ml per 50 kg
Adults:
Body weight Dose
100 kg 7ml
150 kg 10ml
200 kg 14ml
250 kg 17ml
300 kg 20ml
Meat : 28 days


PAXON PLUS - Parvaquone and Furosemide injection
Parvaquone 150 mg
Furosemide B.P 55 mg
Sorbitan Mono Oleate B.P 100 mg
( as preservative )
Oil base q.s.
Parvaquone is theilericidal, acting on schizonts and piroplasms:
Frusemide is a diuretic, which resolves pulmonary oedema.
Inject by the Intramuscular route into the neck muscles at the rate of 1 ml/50 kg (10.0 mg Parvaquone, 3.6 mg Furosemide per kg). Repeat after 48 hours. In case of exceptionally server infections with pulmonary oedema, further treatment at half the dosage rate may be required at 24 hour intervals. Calves : 1 ml/50 kg body weight Young-stock and adults:
100kg 7ml
150kg 10ml
200kg 14ml
250kg 17ml
300kg 20ml
Alternatively. When pulmonary symptoms have been resolved by PAXON PLUS, treatment to achieve parasitic cure may be completed with PAXON which contains only Parvaquone, at a dose rate of 1 ml/50 kg every 48 hours. Do not use by the Intravenous or Subcutaneous route. Normal asceptic precautions should be observed
Meat : 28 days


PROFEN - Ketoprofen 100 mg/ml injection
Ketoprofen 100 mg
Benzyl Alcohol B.P 1% w/v
Water For Injection B.P q.s.
Concurrent administration or administration of other non-steroidal anti-inflammatory drugs within 24 hours.
Administration in animals suffering from cardiac, hepatic or renal disease.
Administration to pregnant mares, because the effects of ketoprofen on fertility, pregnancy or foetal health of horses have not been determined.
Cattle : 1.0 ml per 33 kg body weight by intravenous or deep intramuscular injection once daily for up to 3 days.
Pigs : 1.0 ml per 33 kg body weight once by deep intramuscular injection.
Meat : 4 days
Horse : Meat : 28 day

SELEXIDANT - Vitamin E and Selenium Injection
Vitamin E, α-tocopherol acetate 50 mg
Sodium selenite pentahydrate
equi. To selenium 0.30 mg
Benzyl alcohol B.P 2% v/v
Water for Injection B.P q.s.
Prevention of iron intoxication after administration of iron to Piglets.
Calves, Goats and Sheep :
2 ml per 10 kg body weight, repeat after 2-3 weeks.
Swine :
1 ml per 10 kg body weight, repeat after 2-3 weeks.
For milk : 0 days


SIPHOS-12 - Sodium Acid Phosphate With Vitamin B12 Injection
Sodium acid phosphate B.P 40% w/v
Equi. to Elemental Phosphorous 79.4 mg
Cynocobalamin B.P 50 mcg
Methyl Hydroxybenzoate B.P 0.18% w/v
Propyl Hydroxybenzoate B.P 0.02% w/v
Water for injection B.P q.s.
Folic Acid acts in synergy in the formulation of DNA, and deficiencies can have serious consequences, both in performance horses with a high tissue turnoverA rate, and in pregnancy and growth of young foals.
Clinically the first sign of deficiency is anaemia. Vitamin B12 is essential to the formation of FOLIC ACID. It is useful to stimulate appetite in horses, and is essential maintainance of adequate blood counts.
Small animals : 2-5 ml daily dose can be adjusted according to the phosphorous need of the animal. It is advisable to administer half the dose by s/c route in several places.

THIACIN 500 Thiamine Injection
Thiamine hydrochloride BP 500 mg
(Vitamin B1)
Water for Injection BP q.s.
In any situation where carbohydrates are the major energy source, or when glucose is added to the diet, thiamine requirement is increased significantly.
Thiamine is essential as part of the coenzyme which is involved in the breakdown of glucose for energy. Thiamine, in conjunction with Vitamin B6 (Pyridoxine) is essential in the metabolism of proteins and amino acids, Thiamine has effects on all tissues. The most sensitive are nerves, stomach and heart. Thiamine can not store in the body, and it is rapidly absorbed from the intestine or blood, as well as from injection sites. Like all B Complex vitamins, Thiamine is water soluble, so it is rapidly absorbed and excreted from the body, and requires regular supplementation, especially in hard working animals when dietary input will probably not be sufficient. Thiamine is found in both meat and cereal products. Small amounts are manufactured in the gut, as long as Horses are not under stress. Thiamine in food is destroyed by cooking. High dose of thiamine are reported to help calm nervous or over excitable Horses. Clinical signs of thiamine deficiency include fatigue, muscle weakness, loss of appetite and increased heart rate. (This may be an important factor in endurance Horses fed high grain diets). Many of these signs can be traced back to increased tissue levels of lactic and pyruvic acids. Nerve cells are particularly dependant on carbohydrate metabolism, and normal nerve function is greatly affected by increased levels of these acids during hard work.
Cats : 0.5 to 5 mg
Horses, Cattle and Swine : 100 mg Per 100 kg of body weight.
Administer intramuscularly, once daily Or as recommended by the veterinarian

TROXIN - Ceftriaxone Sodium Powder for Injection
Ceftriaxone Sodium B.P
equi. to Ceftriaxone anhydrous 3 g
Biliary elimination occurs and hence, Ceftriaxone should be given cautiously to those species of animal with extended large intestines e.g. Horse.
TROXIN 3g Injection should be diluted with 10 ml WFL and injected at 12-24 hours interval. It can be administered either intramusculary or intravenously.
Large animals : 7.5 - 25ml / 50kg body weight.
Calf and Goat : 1.5 - 5ml / 10kg body weight.
Dogs : 1.5 - 5ml / 10kg body weight.
Cat : 0.25 - 0.5 ml/kg body weight.
Meat : 28 days

VITCIN - Multivitamin Injection
Vitamin A [palmitate] B.P 10000 I.U.
Thiamine Hydrochloride B.P 50mg
Riboflavin Phosphate Sodium B.P 10 mg
Nicotinamide B.P 100 mg
D-Panthenol USP 25 mg
Pyridoxine Hydrochloride B.P 15 mg
Ascorbic Acid B.P 500 mg
Vitamin E Acetate B.P
[Tocopheryl Acetate B.P] 5 mg
Benzyl Alcohol B.P [as preservative] 1.5% w/v
Water for Injection. B.P Q.S.
-Prevention or treatment of vitamin or amino acid deficiencies in farm animals.
-Prevention or treatment of stress (caused by vaccination, diseases, transport, high humidity, high temperatures or extreme temperature changes).
-Improvement of feed conversion.
Cattle : 10-15 ml.
Goats and Sheep : 5-10 ml.
Swine : 2-10 ml.

VIT-K - Menadione Sodium bisulfite Vitamin k Injection
Menadione sodium bisulfite 10 mg
Water for Injection B.P q.s.
Horses, Cattle : 10 - 12 ml
Calves : 2 - 5 ml
Dogs and Cats : 0.1 - 1 ml
Sheep and Goats : 0.05 - 0.25 ml/kg body weight daily


Water Soluble Powder for Equine (Horse)
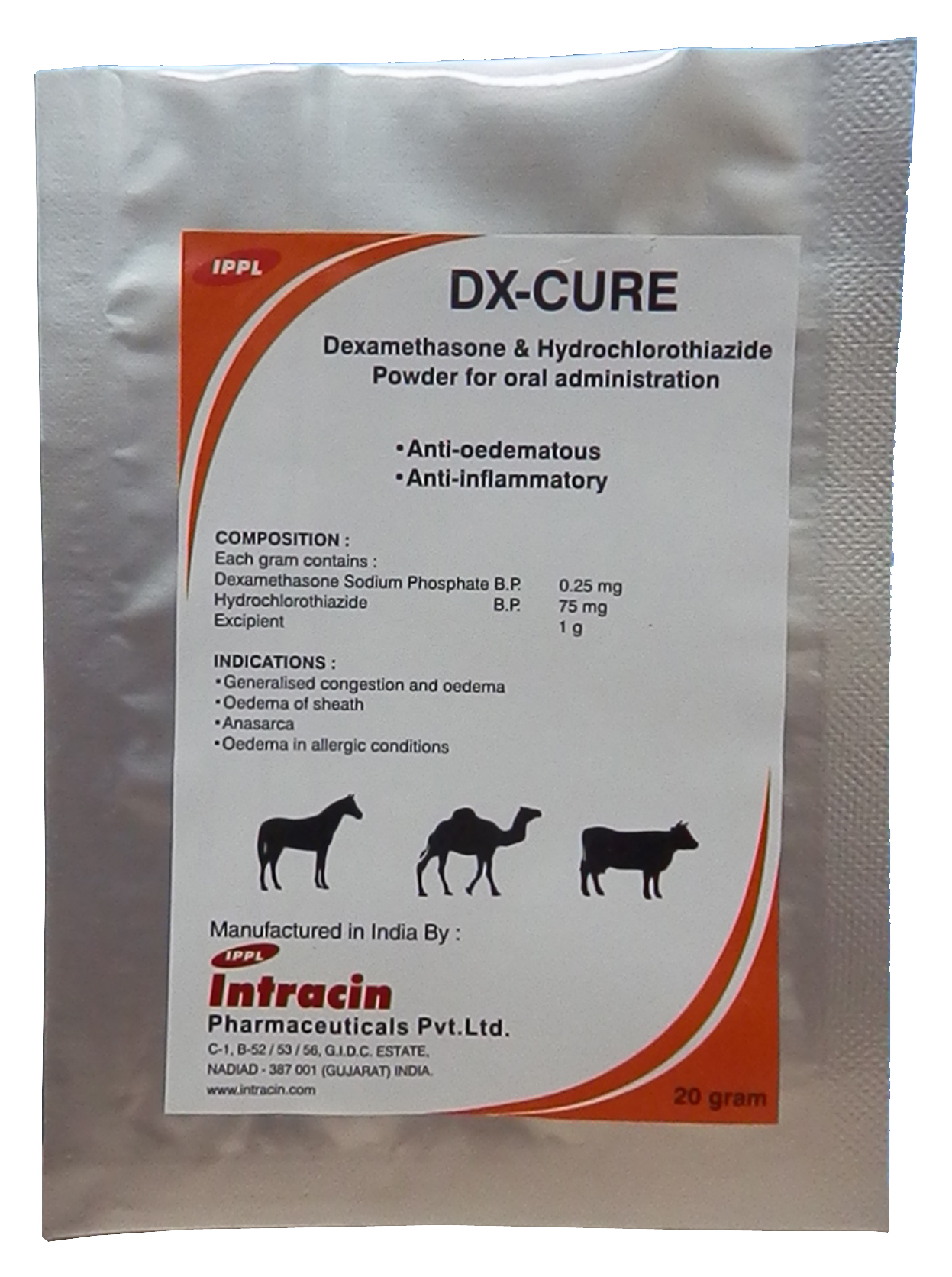
DX-CURE - Dexamethasone & Hydrochlorothiazide Powder
Dexamethasone Sodium Phosphate B.P. 0.25 mg
Hydrochlorothiazide B.P. 75 mg
Excipient 1 g
- Generalised congestion and oedema
- Oedema of sheath
- Anasarca
- Oedema in allergic conditions
- Pregnant animals.
- Animals with viral infections, during the viraemic phase.
- Animals with diabetes mellitus, congestive heart failure, chronic nephritis, osteoporosis or glaucoma.
- Animals with hepatic encephalopathy.
- Cases of severe hypokalaemia.
- Animals with known hypersensitivity to the active ingredients.
Adult Cattle and Horses: 2 sachet on the 1st day. 1 sachet on the 2nd and 3rd days (2 g/ 25-50 kg body weight).
Milk : 3 days.

PIZINE WS - Piperazine Citrate Powder
Piperazine citrate B.P 1000 mg
Swine : Oesophagostomum and Ascaris.
Cattle : Ascaris, Nematodirus, Ostertagia, Cooperia and Oesophagostomum.
Horses : Strongylus, Oxyuris, Trichonema and Ascaris.
Poultry : Ascaris and Capillaria.
- Hypersensitivity to piperazine.
- Administration to animals with a seriously impaired hepatic and/or renal function.
Horses, Swine and Cattle : 1 - 2 g per 10 kg body weight through drinking water.
Poultry : 1 kg per 1000 litres of drinking water for 2 days.
For eggs : 2 days.




